The 510(k) premarket notification process is a critical pathway for medical device manufacturers seeking FDA clearance to market their products in the United States. However, not all 510(k) submissions are created equal. Understanding the different types of 510(k) submissions is essential for navigating this regulatory landscape effectively. In this blog, we’ll explore the various types of 510(k) submissions and shed light on when each type is appropriate.
Traditional 510(k) Submission
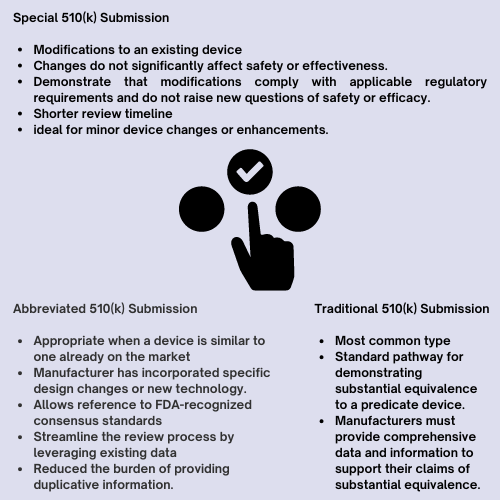
The traditional 510(k) submission is the most common type and follows the standard pathway for demonstrating substantial equivalence to a predicate device. Manufacturers must provide comprehensive data and information to support their claims of substantial equivalence, including device descriptions, performance testing, and risk assessments. The US FDA reviews the submission to determine whether the new device is substantially equivalent to the predicate device in terms of safety and effectiveness.
Abbreviated 510(k) Submission
An abbreviated 510(k) submission is appropriate when a device is similar to one already on the market, but the manufacturer has incorporated specific design changes or new technology. Unlike the traditional pathway, an abbreviated 510(k) allows manufacturers to reference FDA-recognized consensus standards to demonstrate compliance with certain regulatory requirements. This type of submission can streamline the review process by leveraging existing data and reducing the burden of providing duplicative information.
Special 510(k) Submission
A special 510(k) submission is intended for modifications to an existing device that do not significantly affect its safety or effectiveness. Manufacturers must demonstrate that the modifications comply with applicable regulatory requirements and do not raise new questions of safety or efficacy. This type of submission typically has a shorter review timeline compared to traditional or abbreviated 510(k) submissions, making it ideal for minor device changes or enhancements.
Multiple 510(k) Submission
In some cases, manufacturers may submit multiple 510(k) applications for variations of the same device or for multiple indications for use. Each submission must demonstrate substantial equivalence to the appropriate predicate device(s) and provide sufficient data to support the specific intended use(s) of the device. Manufacturers should clearly articulate the differences between each submission and provide rationale for any variations in design, technology, or intended use.
When to choose traditional, abbreviated and Special 510(k) submissions
Choosing between traditional, abbreviated, and special 510(k) submissions depend on the nature of the medical device and the extent of changes or modifications made to it. Here’s a guideline on when to opt for each type:
Traditional 510(k) Submission
- When introducing a new device or technology: If your device is substantially different from any predicate device on the market, a traditional 510(k) submission is typically required. You’ll need to provide comprehensive data to demonstrate substantial equivalence.
- Significant changes or modifications: If your device has undergone significant modifications or changes that may affect its safety or effectiveness, it’s advisable to pursue a traditional 510(k) submission. This includes changes to the device’s intended use, design, materials, or manufacturing process.
Abbreviated 510(k) Submission
- Minor modifications or technological advancements: If your device is similar to an existing device but incorporates minor modifications or technological advancements that do not significantly affect its safety or effectiveness, an abbreviated 510(k) submission may be appropriate.
- Leveraging FDA-recognized consensus standards: If your device conforms to FDA-recognized consensus standards, you can reference these standards in your submission to demonstrate compliance with certain regulatory requirements. This can streamline the review process and reduce the burden of providing extensive data.
Special 510(k) Submission
- Minor changes or enhancements: If your device has undergone minor changes or enhancements that do not raise new questions of safety or efficacy, a special 510(k) submission may be suitable. This includes changes to labeling, manufacturing processes, or materials that do not affect the device’s performance or intended use.
- Expediting review process: If you need to obtain FDA clearance for device modifications quickly, a special 510(k) submission offers a shorter review timeline compared to traditional or abbreviated submissions. This can be beneficial for making minor improvements to existing devices without significant delays.
In summary, choose a traditional 510(k) submission for new devices or significant modifications, an abbreviated 510(k) submission for minor changes or technological advancements, and a special 510(k) submission for minor enhancements or when expediting the review process is crucial. It’s essential to carefully assess the nature and impact of the changes to determine the most appropriate submission pathway and ensure compliance with FDA regulations.
For expert guidance on navigating US FDA 510(k) Pre-Market Notification (PMN) requirements and ensuring successful FDA 510(k) registration, contact us at contact@8chealthcare.com. Our team of regulatory experts is here to support you every step of the way.