US FDA 510(k) Reviews: Handling Deficiency Letters or AI Letters
The 510(k) review process is a critical step for medical device manufacturers seeking
Impact of Design Changes on CDSCO approved Medical Devices
Changes in the design of medical devices can have significant implications on their
Navigating the Current Medical Device Regulatory Scenario in India: Insights from Our Upcoming Webinar
The medical device regulatory landscape in India is undergoing significant changes, presenting both
Substantial Equivalence for Medical Devices for successful US FDA 510(k) registration
For compliance with medical device regulation in the United States, demonstrating substantial equivalence
Choosing the Right Type of US FDA 510(k) Premarket Notification for Your Medical Device: Understanding the Options
The 510(k) premarket notification process is a critical pathway for medical device manufacturers
Creating an Account in the SUGAM Online Portal and Leveraging the National Single Window System
In India, the SUGAM online e-Governance portal facilitates the submission, review, and approval
Impact of Post Approval Changes in Constitution of the Indian Authorized Agent (IAA) or Manufacturer on India Medical Device Approvals
Navigating the regulatory framework surrounding medical devices in India requires meticulous attention to
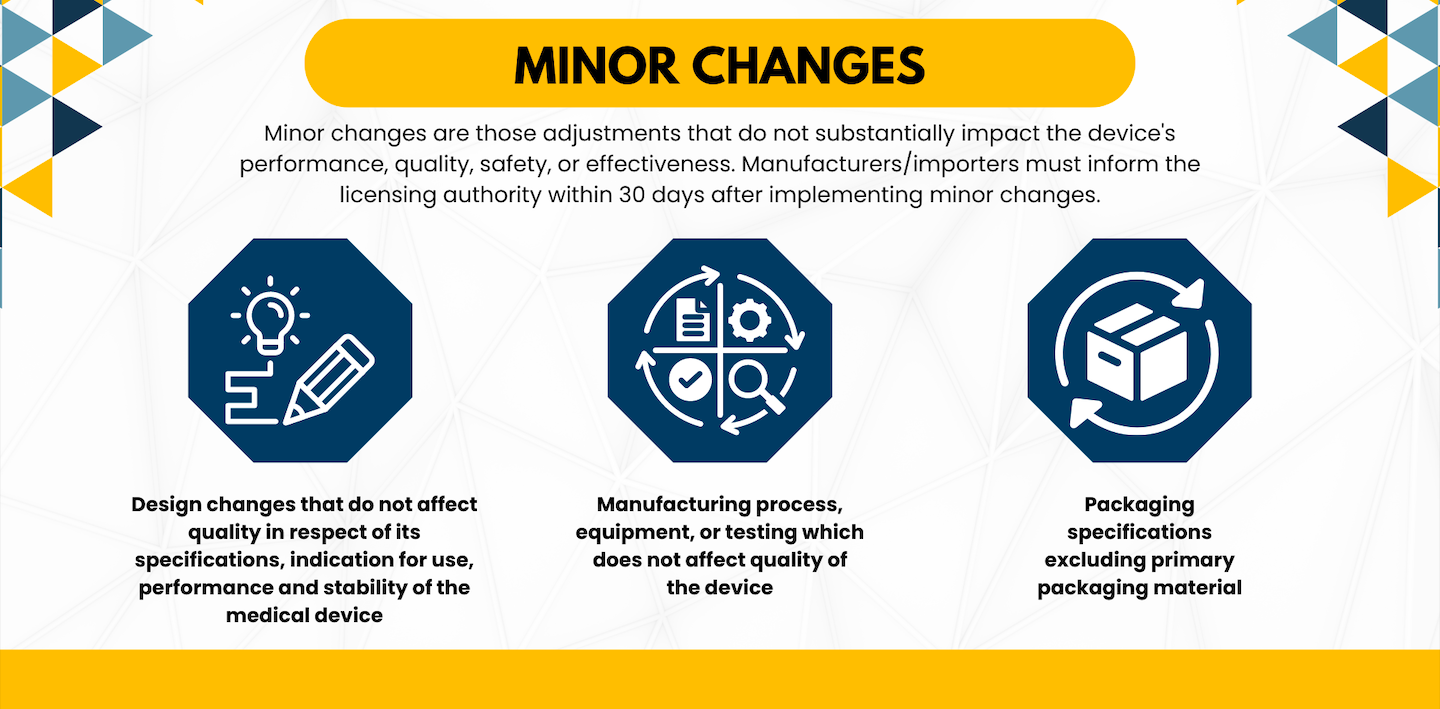
Post Approval Change Notifications and Their Intimation Timelines
In the ever-evolving landscape of medical device regulations in India, manufacturers and importers
Online application for Obtaining Neutral code for Medical Devices Manufactured for Export purpose
On February 12, 2024, the Central Drugs Standard Control Organization unveiled a notable
US FDA 510(k) Pre-Market Notification (PMN) Submission requirements for Sterile Medical Devices
Sterilization technologies have undergone a remarkable evolution over the years, driven by advancements