
The Device Master File (DMF) is a comprehensive document that provides detailed information about a medical device. It is a critical component of the medical device registration process in India, and is required for all Class A (Measuring and Sterile), Class B, Class C, and Class D medical devices. It provides the CDSCO with comprehensive information about the medical device, which allows them to efficiently review registration applications and assess the safety and efficacy of the device. It serves as a central repository of information about medical devices, which can be used for other regulatory purposes, such as post-market surveillance and enforcement.
The DMF is a valuable resource for the CDSCO, as it allows them to efficiently review medical device registration applications. It also provides a central repository of information about medical devices, which can be used for other regulatory purposes, such as post-market surveillance and enforcement.
The DMF is used by the Central Drugs Standard Control Organization (CDSCO) to assess the safety and efficacy of medical devices. It contains information such as:
- Device description and specifications
- Manufacturing process and quality control procedures
- Design and development documentation
- Risk management documentation
- Clinical trial data
- Biocompatibility and safety data
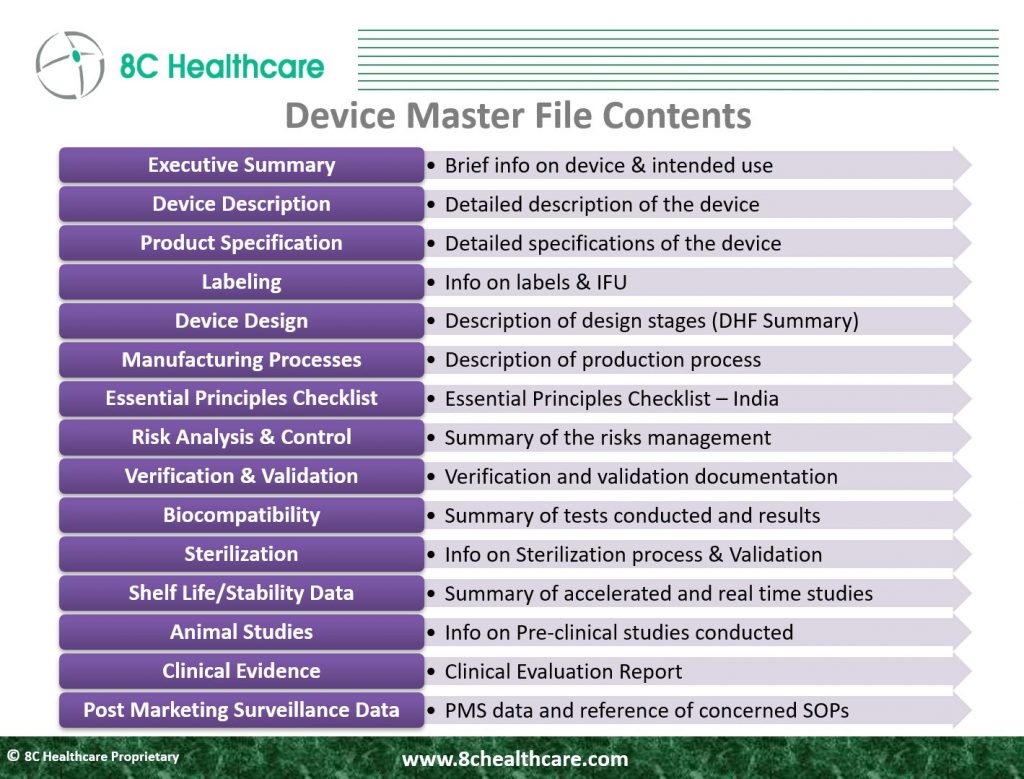
Key components of the Device master file
The key components of a Device Master File (DMF) for medical devices in India are:
- Executive Summary: The executive summary offers a concise overview of the DMF, summarizing crucial details within. It provides a brief description of the medical device, its intended use, and highlights significant aspects essential for regulatory approval.
- Device Description and Product Specifications: This section furnishes in-depth information about the device, including technical specifications. It covers descriptive details, product specifications, references to the predicate, materials, construction, and specific features. Accurate specifications are vital for regulatory clearance.
- Labelling: Labelling information in the DMF adheres to Chapter VI requirements. It encompasses product label design, usage instructions, warnings, and other end-user information.
- Design and Manufacturing Information: This outlines the device’s design and manufacturing processes. It includes detailed schematics, manufacturing flowcharts, process descriptions, and quality control measures involved in device usage.
- Essential Principle Checklist: This checklist demonstrates the device’s alignment with essential safety and performance principles outlined by Indian regulations. It showcases how the device design and performance meet the standards set by CDSCO, ensuring safety and efficacy.
- Risk Analysis and Control Summary: This section assesses potential device risks and outlines strategies to mitigate them. Identifying hazards and implementing effective risk control measures are crucial for ensuring device safety throughout its lifecycle.
- Verification and Validation: These processes confirm the device’s adherence to design specifications and functionality. It varies in the level of detail as determined by the class of the device. It includes Summary information, analytical studies, specimen type, and Verification to ensure that each design phase meets its requirements.
- Clinical Evidence: This section provides data from clinical studies supporting the device’s safety and efficacy. Detailed reports on investigations, trials, or studies validate the device’s performance and impact on patient health.
- Biological Safety: The documentation should provide a detailed list of materials obtained from animals or humans for the device, including information on source selection, harvesting, processing, testing, and handling. It must also include process validation results to show that manufacturing minimizes biological risks, especially related to viruses and other transmissible agents. Certificates for Transmissible Spongiform Encephalopathies (TSE) or Bovine Spongiform Encephalopathy (BSE) are necessary for submission.
- Sterilization: For sterile devices, documents must detail sterilization validation, including sterilizer qualification, bioburden, pyrogen, and residue testing. All tests must adhere to standards, with clear methods, sterility levels, and results summary provided
- Stability Data: If real-time aging data is accessible, it should support the claimed shelf life. If not, accelerated stability data can be submitted initially, with real-time testing starting promptly. After real-time analysis, confirmed stability data should be provided to validate the stated shelf life.
- Animal Studies: when animal studies are conducted to ensure safety and performance, the dossier should offer comprehensive data. This should cover study goals, methodologies, findings, analysis and conclusion while maintaining a commitment to Good Laboratory Practices. It should also explain the choice of animal and acknowledge any known limitations.
- Post- Market Surveillance: The dossier should include the manufacturing procedures and collected data related to Post- Market Surveillance. This data should encompass compliant details, corrective and the preventive measures taken.
Overall, the DMF is a significant document in the Indian medical device registration process. It plays an important role in ensuring the safety and efficacy of medical devices in India.
At 8chealthcare, our seasoned experts specialize in Indian Medical Devices regulations, providing comprehensive support from DMF compilation to submission. Reach out to 8chealthcare today, and allow us to be your trusted partners in ensuring compliance, expediting your approvals, and facilitating your seamless entry into the Indian market.











