In the ever-evolving landscape of medical device regulations in India, manufacturers and importers must navigate the intricacies of post-approval changes with diligence and adherence to regulatory requirements. Understanding the obligations surrounding these changes is paramount to ensuring continued compliance and maintaining the safety and effectiveness of medical devices in the market.
What are Post Approval Changes?
Post approval changes refer to any alterations made to a medical device, its manufacturing process, or the manufacturing facility subsequent to obtaining approval from the Central Drugs Standard Control Organization (CDSCO). These changes have the potential to impact the device’s performance, quality, safety, and effectiveness, necessitating regulatory oversight and notification to the licensing authority.
Categorization of Changes: Major vs. Minor
Post approval changes are classified into two categories: major changes and minor changes.
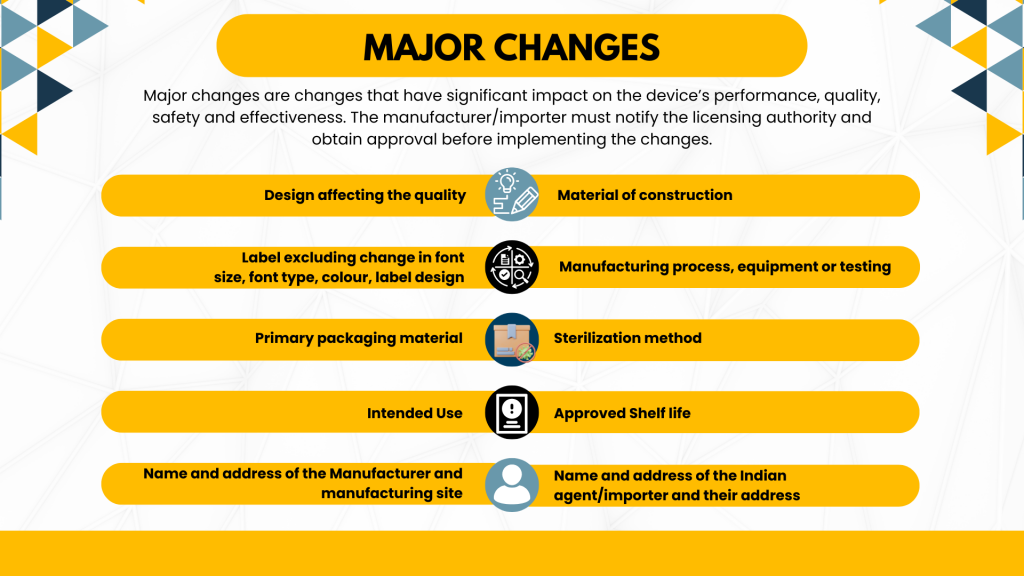
Major Changes
Major changes are those alterations that significantly affect the device’s performance, quality, safety, or effectiveness. Examples include modifications to the material of construction, design affecting quality, intended use, sterilization method, approved shelf life, labeling (excluding minor alterations), manufacturing process, equipment, or primary packaging material. Manufacturers/importers must notify the licensing authority and obtain approval before implementing major changes.
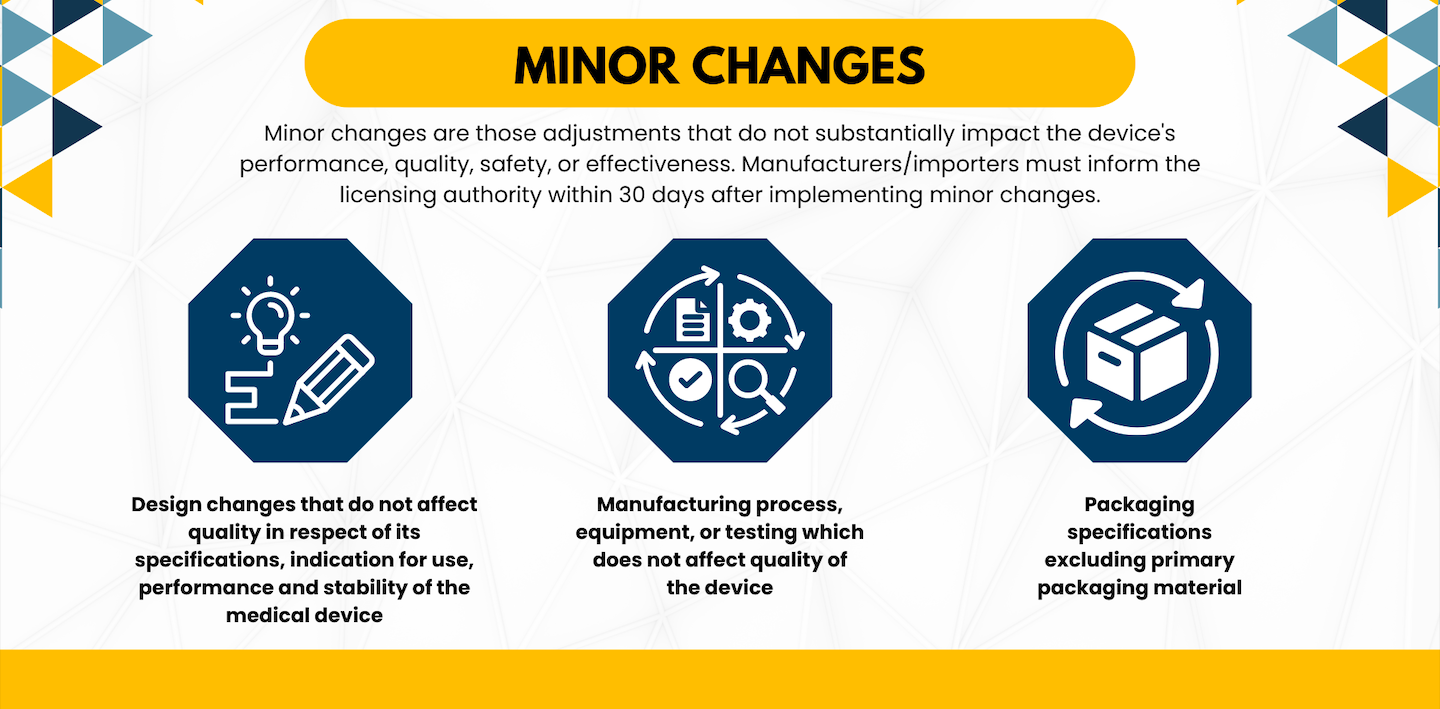
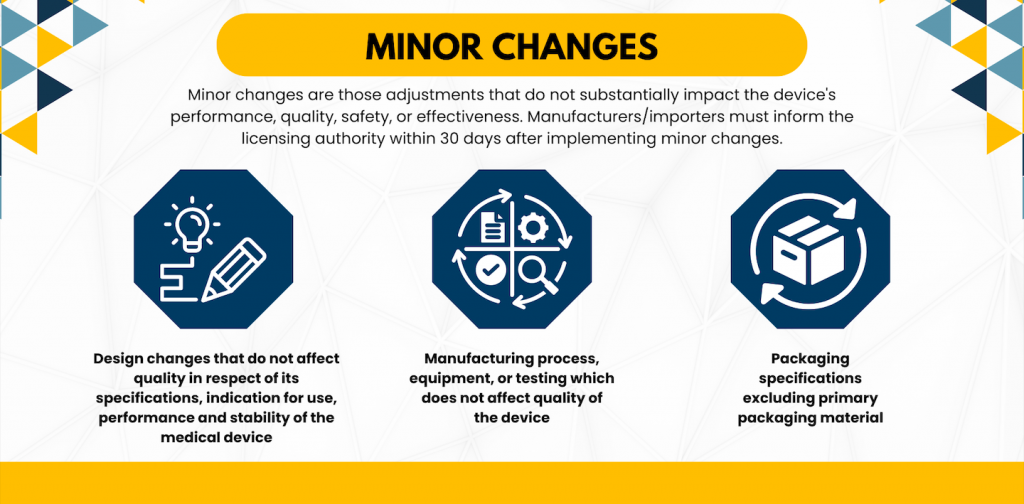
Minor Changes
Minor changes are those adjustments that do not substantially impact the device’s performance, quality, safety, or effectiveness. This category encompasses alterations to design specifications, manufacturing processes/equipment/testing (that do not affect device quality), and packaging specifications (excluding primary packaging material). Manufacturers/importers must inform the licensing authority within 30 days after implementing minor changes.
Compliance and Regulatory Requirements
Adherence to post approval change notification requirements is crucial for manufacturers/importers to maintain regulatory compliance and ensure the continued safety and effectiveness of medical devices. By notifying the licensing authority of any changes promptly and following the prescribed processes, manufacturers can facilitate efficient regulatory oversight and contribute to the enhancement of device performance and quality.
Series on Post Approval Change Notifications
At 8C Healthcare Pvt. Ltd., we recognize the significance of post approval change notifications in the medical device industry. We are committed to providing valuable insights and guidance on navigating the regulatory landscape surrounding these changes. Stay tuned for our upcoming series of posts, where we will delve into various scenarios of post approval change notifications and their regulatory requirements.
Already Available Blogs:
- Impact of Change in Address of the Foreign Manufacturer or Its Manufacturing Site
- Impact of Change in Intended Use/Indication of the Device
Need Assistance?
If you are a manufacturer contemplating changes to your medical device and require clarity on the notification process for post approval changes, our team at 8C Healthcare Pvt. Ltd. is here to assist you. Feel free to reach out to us at [Insert Contact Information] for personalized guidance and support.
Let’s navigate post approval changes together and uphold the highest standards of quality and safety in the medical device industry.
#MedicalDevices #RegulatoryCompliance #PostApprovalChanges #CDSCO #India #IVD #Digital Health