𝗡𝗮𝘁𝗶𝗼𝗻𝗮𝗹 𝗣𝗼𝗹𝗶𝗰𝘆 𝗼𝗻 𝗥𝗲𝘀𝗲𝗮𝗿𝗰𝗵 & 𝗗𝗲𝘃𝗲𝗹𝗼𝗽𝗺𝗲𝗻𝘁 𝗮𝗻𝗱 𝗜𝗻𝗻𝗼𝘃𝗮𝘁𝗶𝗼𝗻 𝗶𝗻 𝘁𝗵𝗲 𝗣𝗵𝗮𝗿𝗺𝗮-𝗠𝗲𝗱𝗧𝗲𝗰𝗵 𝗦𝗲𝗰𝘁𝗼𝗿
October 10, 2023
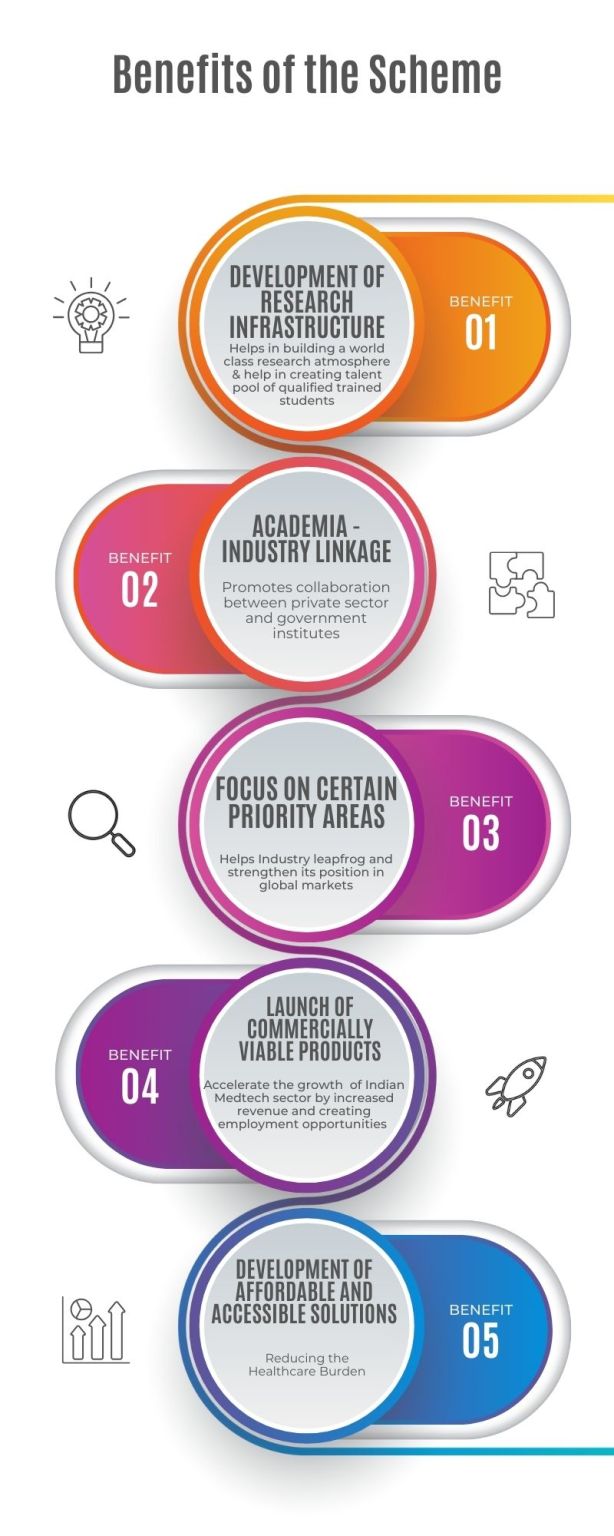
The Indian government approved the 𝗡𝗮𝘁𝗶𝗼𝗻𝗮𝗹 𝗣𝗼𝗹𝗶𝗰𝘆 𝗼𝗻 𝗥𝗲𝘀𝗲𝗮𝗿𝗰𝗵 & 𝗗𝗲𝘃𝗲𝗹𝗼𝗽𝗺𝗲𝗻𝘁 𝗮𝗻𝗱 𝗜𝗻𝗻𝗼𝘃𝗮𝘁𝗶𝗼𝗻
Licensing Now Essential for all Medical Devices and IVDs
October 10, 2023
Important Update on the Medical Device Industry in India. Class C and Class
Impact of change in address of the foreign manufacturer or its manufacturing site
August 29, 2023
In India, the manufacturing sites are used to determine whether to grant a manufacture
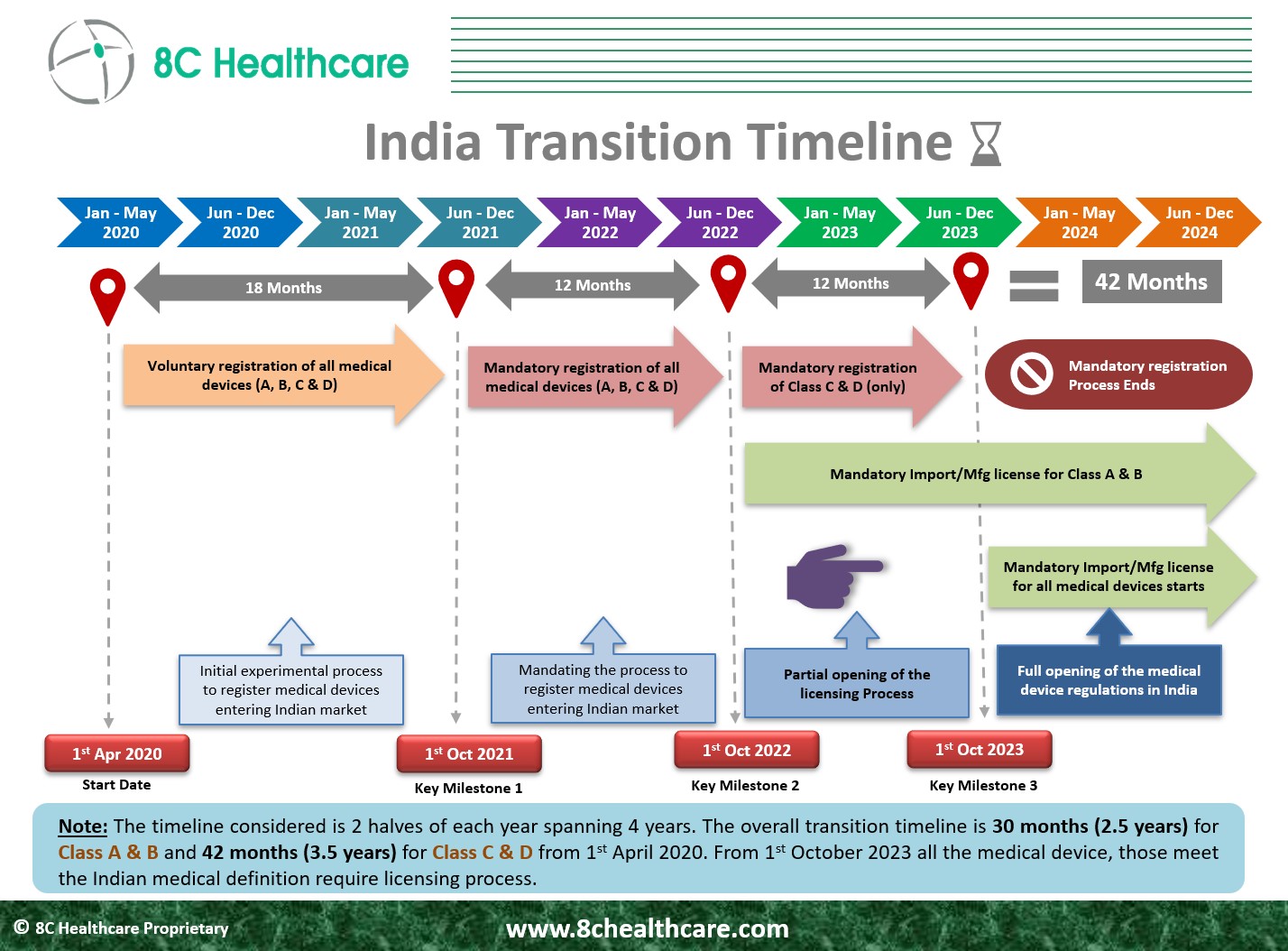
Regulation of all medical devices in India by Oct 1, 2023
August 29, 2023
Starting October 1, 2023, 𝒂𝒍𝒍 𝒏𝒐𝒏-𝒏𝒐𝒕𝒊𝒇𝒊𝒆𝒅 𝑪𝒍𝒂𝒔𝒔 𝑪 𝒂𝒏𝒅 𝑪𝒍𝒂𝒔𝒔 𝑫 𝑴𝒆𝒅𝒊𝒄𝒂𝒍 𝒅𝒆𝒗𝒊𝒄𝒆𝒔
Obtaining a Loan License for Medical Devices in India from CDSCO
August 29, 2023
The medical device sector in India is witnessing remarkable growth and innovation, making
Class C & D Medical Devices
April 26, 2023
It is now prominent that in order to maintain market access starting on
Classification and Grouping of Medical Devices & IVDs in India
April 26, 2023
Medical devices and In Vitro Diagnostic (IVD) products play a crucial role in
Registration of Class C and D devices in India
February 8, 2023
According to the new medical device Rules, 2017, there are 4 categories of