According to the new medical device Rules, 2017, there are 4 categories of medical devices – Class A, Class B, Class C and Class D. Medical devices having minimal risk are classified as Class A and those having moderate risk are classified as Class B devices. For Class A and B devices, the Licensing Authority of the respective Indian state in which the device is manufactured shall receive and process applications from manufacturers for grant of a manufacturing license. Devices which pose a high risk are classified as Class C and those posing very high risk are classified as Class D devices. The Central Licensing Authority (CDSCO) shall receive and process applications from manufacturers of Class C and D devices for grant of a manufacturing license. The CDSCO shall receive and process the applications for the grant of Import License (for import of devices into India) for all device classes (A, B, C and D).
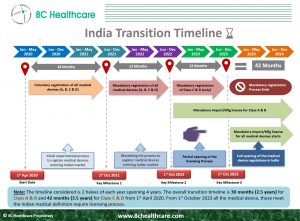
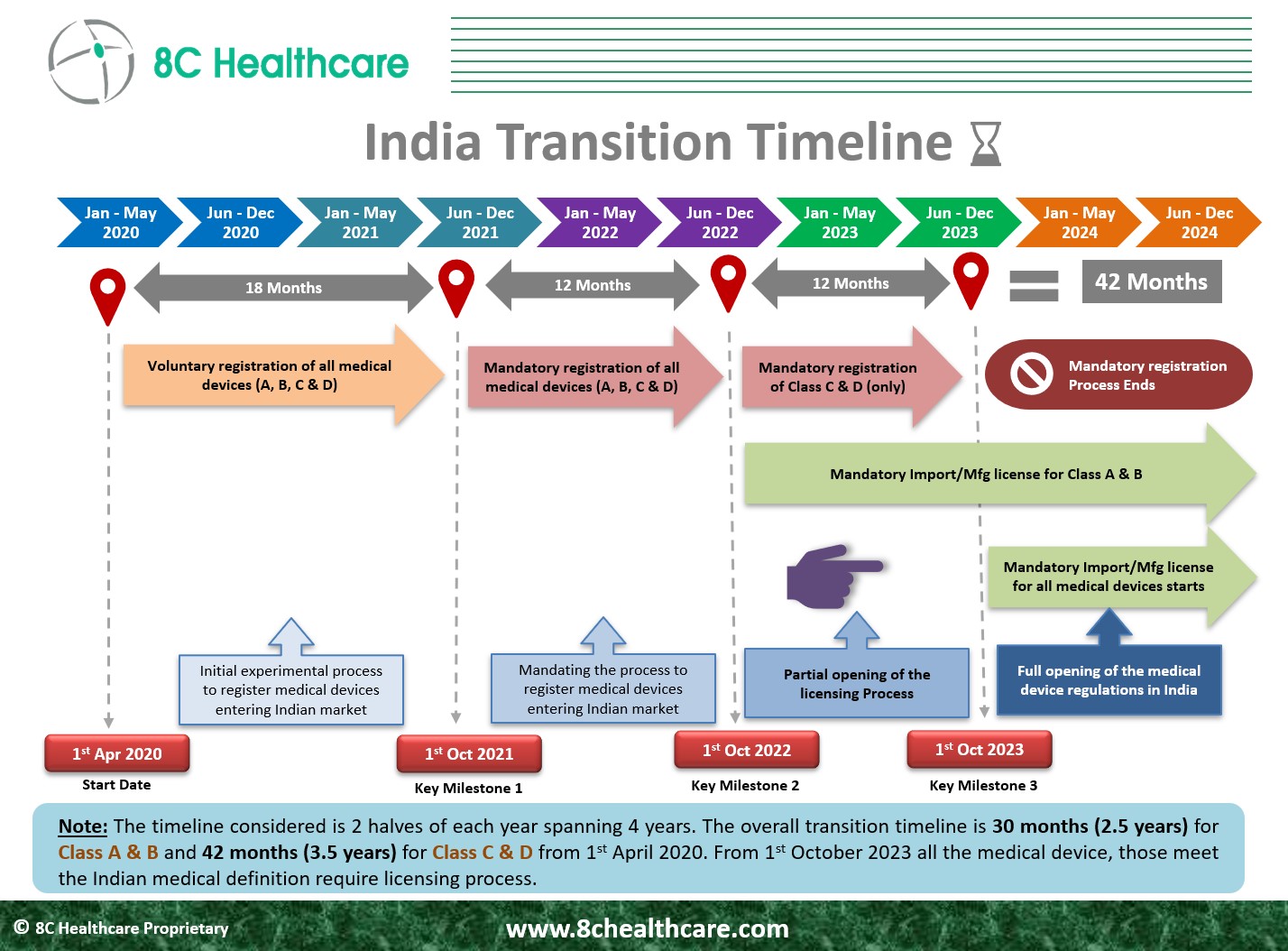
A transition period of 30 months was provided for Class A and Class B devices from the date of implementation of the New Medical Device (Amendment) Rules 2020 (G.S.R. 102(E) i.e., 01.04.2020) for the transition to licensing regime. All Class A and B devices were brought into the Licensing Regime from 1st October 2022. A transition period of 42 months was provided for Class C and Class D devices from the date of implementation the New Medical Device (Amendment) Rules 2020 (G.S.R. 102(E) i.e., 01.04.2020) for the transition to licensing regime. All Class C and D devices will be brought into the Licensing Regime from 1st October 2023.
All non-notified Class C and Class D medical devices are currently subject to a mandatory online registration process valid until the 1st of October 2023, and are referred to as “Non-Regulatory Medical Devices.” The registration process shall generate a file number on the day of registration and the importer must mention the file number on the label of the medical device prior to placing the devices on the market. From 1st of October 2023, all Class C and Class D devices shall transition to the Licensing regime.
Considering the 5-6 month period for CDSCO to review the application and grant the Manufacturing/Import Licenses, in order to have a License in hand by October 1, 2023 for Class C and D devices, the application for Manufacturing/Import Licenses need to be submitted to the CDSCO by the end of March 2023. Therefore, it is suggestable to all the medical device Manufacturers to start compiling the applications for Manufacturing/Import Licenses for Class C and D devices in order to continue to market their devices in India.