The medical device sector in India is witnessing remarkable growth and innovation, making it an enticing prospect for entrepreneurs and businesses. However, one of the significant hurdles is the substantial investment required for manufacturing infrastructure. To circumvent this, manufacturers can utilize the facilities of another company that already possesses the infrastructure to produce the desired medical devices. Before you can embark on this journey, it’s imperative to have a solid grasp of the regulatory framework laid out by the Central Drugs Standard Control Organization (CDSCO). This blog is aimed at providing a comprehensive guide on how to secure a loan license for medical devices from CDSCO.
The year 2017 saw the introduction of the Medical Devices Rules, which laid out the framework for establishing and implementing the rules and regulations governing medical devices and in-vitro diagnostic (IVD) devices in India. For a medical device manufacturer in India to produce medical devices, it is mandatory to obtain a manufacturing license from the relevant licensing authority. The same holds true when applying for a loan license for medical devices. This specialized license permits an entity to manufacture, import, distribute, or sell medical devices on behalf of the owner or manufacturer, ensuring adherence to established standards and regulations. However, the licensee must select a manufacturer who already possesses a manufacturing license to apply for a loan license.
As the name suggests, this license is essentially a request to a manufacturer for a loan of an active manufacturing license. It is granted to an entity that intends to utilize the facilities of an existing license holder for the production of medical devices, provided that the said license holder is manufacturing the same category of medical device at the facility.
For manufacturers who possess a manufacturing license but lack a sterilization facility, it is imperative to acquire a loan license for medical devices in accordance with Rules 20 and 21 of the Medical Devices Rules, 2017.
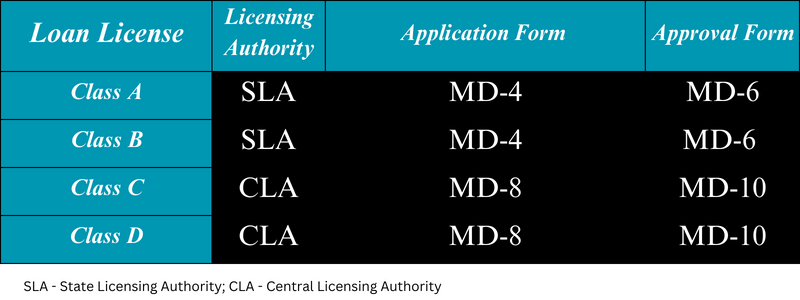
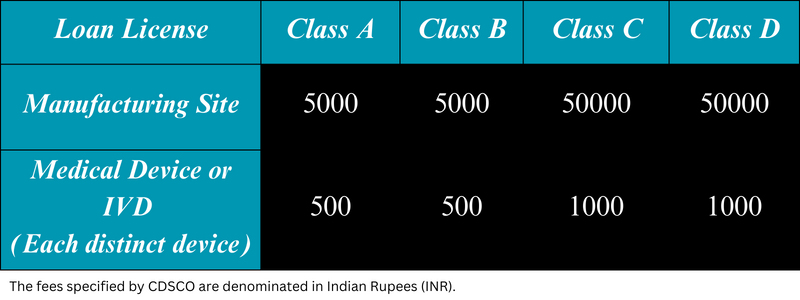
The issuance of loan licenses for Class A and Class B medical devices falls under the jurisdiction of the State Licensing Authority (SLA), while the Central Licensing Authority (CLA) is responsible for issuing loan licenses for Class C and Class D medical devices. Once applications for loan licenses for Class A and Class B medical devices are submitted in Form MD-4, the SLA issues a loan license approval in Form MD-6. Similarly, the CLA grants permission for a loan license in Form MD-10 upon receiving applications for Class C and Class D medical devices in Form MD-8. It’s important to note that the application must be accompanied by the relevant fees.
Prior to seeking a loan license, there are certain prerequisite compliance requirements that must be met, including:
- Adherence to local Quality Management System (QMS) requirements for medical devices at the manufacturing site.
- Appointment of qualified technical personnel to oversee and direct the manufacturing process.
- Appointment of qualified technical personnel with a minimum of two years of expertise in medical device testing. Their guidance and supervision in testing activities play a pivotal role in the manufacturing process.
The validity of loan licenses issued for Class A, B, C, and D medical devices remains indefinite, provided that the license maintenance fee is paid before the expiration of the five-year period from the date of issue, unless it is suspended or canceled by the SLA/CLA. The maintenance fees is same as the initial licensing fees.
For more information on the procedure to make the application and documents required for obtaining a Medical Device Loan License, you can reach out to contact@8chealthcare.com.