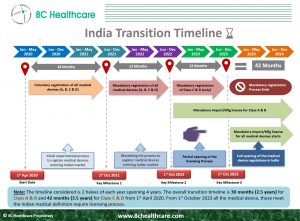
Medical devices and In Vitro Diagnostic (IVD) products play a crucial role in the healthcare industry, assisting healthcare professionals in diagnosing, monitoring, and treating patients. To ensure the safety and effectiveness of these devices, governments worldwide classify and regulate them. The regulation of medical devices and IVDs in India falls under the purview of the Central Drugs Standard Control Organization (CDSCO), which operates under the Ministry of Health and Family Welfare. The primary regulatory framework for these products is governed by the Medical Devices Rules, 2017, which were introduced to strengthen the existing regulatory framework and align it with international standards.
The classification and grouping of medical devices and IVDs in India have several significant implications:
- Regulatory Compliance: Proper classification ensures that manufacturers and importers follow the appropriate regulatory pathway, meeting the required standards and quality control measures.
- Patient Safety: Stringent classification helps in identifying and regulating high-risk devices, minimizing the potential harm to patients and ensuring that only safe and effective products are available in the market.
- Innovation and Research: The regulatory framework encourages innovation and research in the medical device and IVD industry by offering different pathways based on risk. This helps in fostering the development of advanced healthcare technologies.
- Market Access: Manufacturers can navigate the regulatory requirements more effectively when they know the classification of their product, enabling easier market access.
- International Trade: Compliance with the classification system is essential for Indian manufacturers to export their products to international markets, promoting global competitiveness.
Classification of Medical Devices
In India, medical devices are classified into four categories based on their intended use, risk level, and complexity. This classification is crucial for determining the regulatory pathway and requirements for market authorization:
- Class A: Low-risk devices such as bandages, gloves, and walking aids
- Class B: Moderate-risk devices such as surgical instruments, diagnostic equipment, and syringes
- Class C: High-risk devices such as implantable devices, cardiac stents, and ventilators.
- Class D: Very high-risk devices such as drug-eluting stents and heart valves.
Classification of IVDs
IVDs are also classified into four categories based on their risk and intended use:
- Self-certified IVDs: These are low-risk IVDs, like pregnancy test kits, for which manufacturers can self-certify compliance with regulatory requirements.
- List A IVDs: Moderate-risk IVDs listed by CDSCO that require a registration certificate for marketing.
- List B IVDs: Higher-risk IVDs listed by CDSCO, such as HIV test kits. Manufacturers must obtain a registration certificate and comply with more stringent requirements.
- List C IVDs: Very high-risk IVDs, such as blood glucose monitoring systems. These require a registration certificate, and in some cases, clinical performance evaluation data may be necessary.
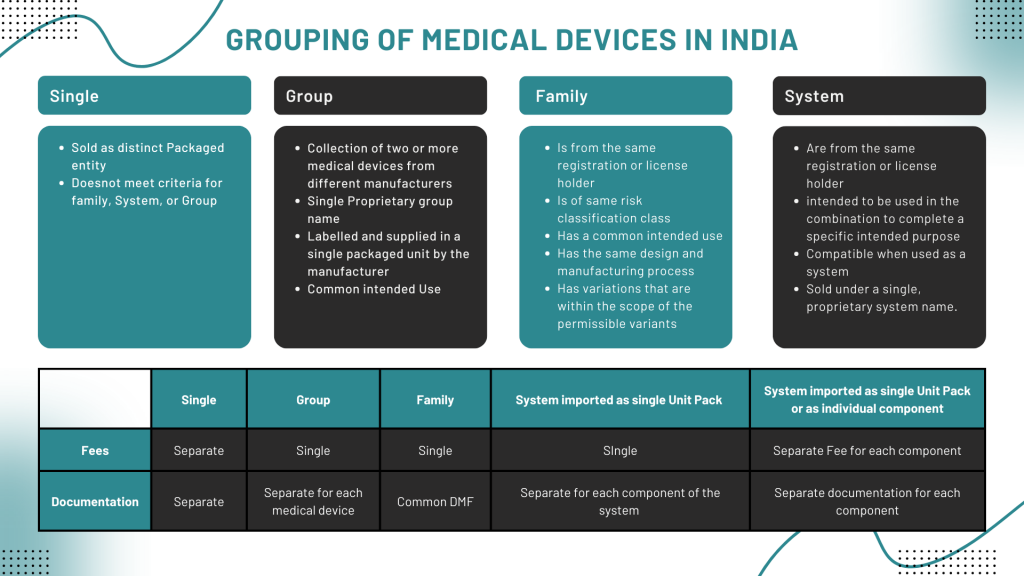
Grouping of Medical Devices
The applicant has the option to consolidate medical devices with identical or similar intended purposes or shared technological features into a single application. This grouping of medical devices serves the purpose of submitting a unified application for licensing, whether for importation or manufacturing. Grouping helps streamline the regulatory process by allowing similar devices to be evaluated together.
The medical devices, their components and variants can be grouped into –
- Single Medical Device
- Medical Device Family
- Medical Device Group
- Medical Device System
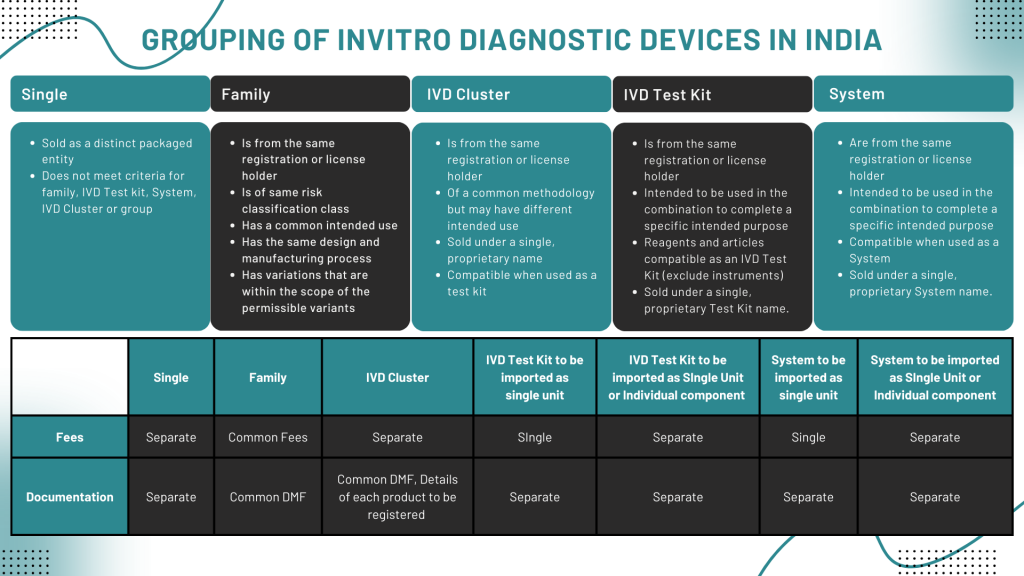
Grouping of In-Vitro Diagnostic Medical Devices
The IVDs can be grouped into any of the following –
- Single
- Family
- IVD Cluster
- IVD Test Kit
- System
The classification and grouping of medical devices and IVDs in India are vital components of the regulatory framework that ensure the safety, quality, and efficacy of these products. It plays a significant role in guiding manufacturers, importers, and regulators in the appropriate evaluation and approval processes. A well-defined classification system not only protects the health and safety of patients but also fosters innovation and promotes the growth of the medical device and IVD industry in India, contributing to the overall advancement of healthcare
If you are a manufacturer, having devices with multiple components and looking for someone to guide and assist you in categorizing and grouping of your medical devices, please feel free to reach out to us at contact@8chealthcare.com.