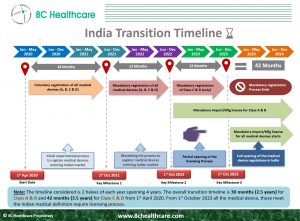
Important Update on the Medical Device Industry in India.
Class C and Class D devices fall under the regulatory scrutiny of CDSCO and now require mandatory registration.
Starting now, ALL medical devices and in vitro diagnostics (IVDs) in India must obtain licenses before they hit the market.
Stay ahead of the curve, ensure compliance, and bring your innovative MedTech solutions to the Indian market. Get in touch with us for expert guidance and support.
#MedicalDevices #CDSCO #HealthcareRegulations #ComplianceMatters #ClassCMedicaldevices #CLassDmedicaldevices #compliance