A Predicate Device in the context of a 510(k) submission refers to a legally marketed medical device in the United States that is used as a reference or comparison when seeking clearance from the U.S. Food and Drug Administration (FDA) for a medical device. When a manufacturer wants to introduce a new medical device to the market, they can submit a 510(k) Pre-Market Notification (PMN) to demonstrate that the new device is substantially equivalent to a legally marketed predicate device.
Key considerations for predicate device identification for 510(k) submissions:
Identifying the appropriate predicate device for a 510(k) submission is a critical step in the regulatory process for medical devices in the United States. Here are the key considerations to help you identify a suitable predicate device:
- Understand Your Device: Have a clear understanding of your medical device, including its intended use, design, technology, and indications for use. Knowing your device’s characteristics will help you search for an appropriate predicate.
- FDA’s 510(k) Database: The FDA maintains a publicly accessible database that contains information about previously cleared 510(k) submissions. You can use this database to search for potential predicate devices. Here’s how to do it:
- Visit the FDA’s website.
- Use the search options provided to filter devices by product code, device name, manufacturer, and other relevant criteria.
- Review the results to find devices that are similar to yours in terms of intended use, technology, and indications.
- Consult FDA Guidance Documents: The FDA provides guidance documents and resources that can help you in your search for a predicate device. These documents often offer recommendations for identifying suitable predicates based on the type of medical device you are developing.
- Seek Professional Guidance: Consider consulting with regulatory experts, consultants, or legal professionals who specialize in medical device submissions. They can provide valuable insights and assistance in identifying the most appropriate predicate device.
- Analyze Predicate Device Characteristics: Once you’ve identified potential predicate devices, carefully review their characteristics and compare them to your device. Look for similarities in intended use, design, technology, and indications for use.
Structured Approach to Predicate Device Evaluation
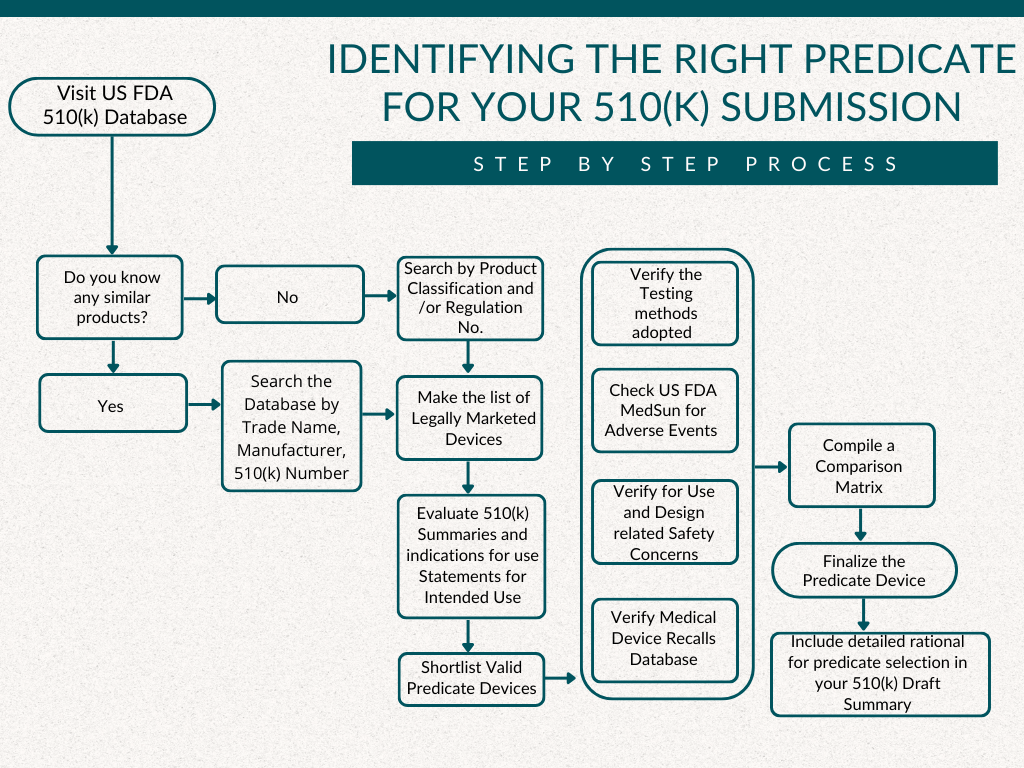
A comprehensive process is to be followed for regulatory adherence and the selection of the most suitable predicate device for your medical device.
- The presence of any similar devices in the market should be investigated in the US FDA 510(k) Database. Potential matches should be meticulously filtered by searching the database using criteria like Trade Name, Manufacturer, and 510(k) Number.
- In instances where no similar devices are found, a refined search based on Product Classification / Regulation Number must be conducted.
- With a list of legally marketed devices in hand, 510(k) summaries and statements need to be assessed to determine their intended use. This meticulous evaluation process allows valid predicate devices to be shortlisted, ensuring alignment with your device’s characteristics.
- Testing conducted on these potential predicates should be diligently verified to confirm their suitability. A preferred predicate device is one cleared with tests conducted using well-established methods developed within consensus standards or other widely accepted methods published in the public domain or scientific literature, and those subject to public comment or peer review.
- In the next phase, safety concerns related to the selected devices must be checked in the Medical Product Safety Network (MedSun), prioritizing the safety of our product. This involves considering the potential role of any reported medical device-related adverse events, malfunctions, or deaths in establishing the safety and effectiveness of the device.
- Verification is necessary to determine if the predicate device has any associated use-related or design-related safety issues. Emerging signals and safety communications published by the US FDA from time to time must be checked, and the safety communication issued for medical devices and Biologics (if applicable) must be verified.
- Additionally, cross-referencing should be done to ascertain whether the predicate device has been subjected to any design-related recalls. The US FDA Medical Devices Recalls database can be searched using Product Name, Product Code, or 510(k) Number.
- After thorough scrutiny, a comprehensive comparison matrix needs to be compiled, enabling the objective evaluation of the strengths and weaknesses of each potential predicate device.
| Valid Predicate Device | Testing Methods Adopted | Adverse Events | Use or Design Related Safety Issues | Design related Recalls |
| A | ||||
| B | ||||
| C | ||||
| D |
- Finally, with the knowledge gathered through this rigorous process, the most appropriate predicate device is confidently selected, thereby setting the stage for a successful 510(k) submission
It’s important to note that choosing the right predicate device is a critical step in the 510(k) submission process. Manufacturers should conduct thorough research and due diligence to select an appropriate predicate device that closely matches their new device’s characteristics.
Additionally, the regulatory landscape and requirements may change over time, so it’s advisable to consult with regulatory experts or legal professionals with expertise in medical device submissions to ensure compliance with current FDA regulations and guidelines.
It’s important to conduct a thorough and diligent search for a suitable predicate device as this choice will significantly impact the success of your 510(k) submission. Additionally, be mindful that FDA regulations and guidance may change over time, so it’s crucial to stay current with the latest requirements and seek professional guidance when needed.
Your commitment to the 510(k) Notification process isn’t just about ticking boxes; it’s about making a difference in the lives of countless patients. Your choices today will shape the healthcare landscape of tomorrow. Make them wisely.
To embark on your journey to regulatory compliance and successful 510(k) submission, take the first step today. Contact our experienced regulatory service experts today. We’re here to guide you through every step of the journey and help you make informed decisions. Don’t leave compliance to chance – reach out now to secure the future of your medical device.