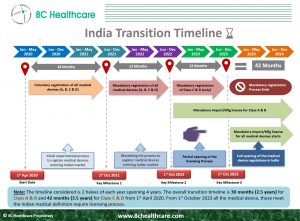
In India, the registration of overseas medical devices and In Vitro Diagnostics (IVDs) is a crucial step to ensure the quality and reliability of these products in the Indian market. The Central Drugs Standard Control Organization (CDSCO) is the regulatory body responsible for overseeing and granting import licenses for these essential healthcare tools. In this blog, we will explore the process of registering overseas medical devices and IVDs in India and obtaining the necessary import license from the CDSCO
Commencing on October 1, 2023, all foreign manufacturers are required to secure pre-market approval from the CDSCO prior to the importation of medical devices and IVDs into India. Following a thorough review of the application, the CDSCO will then grant an import license.
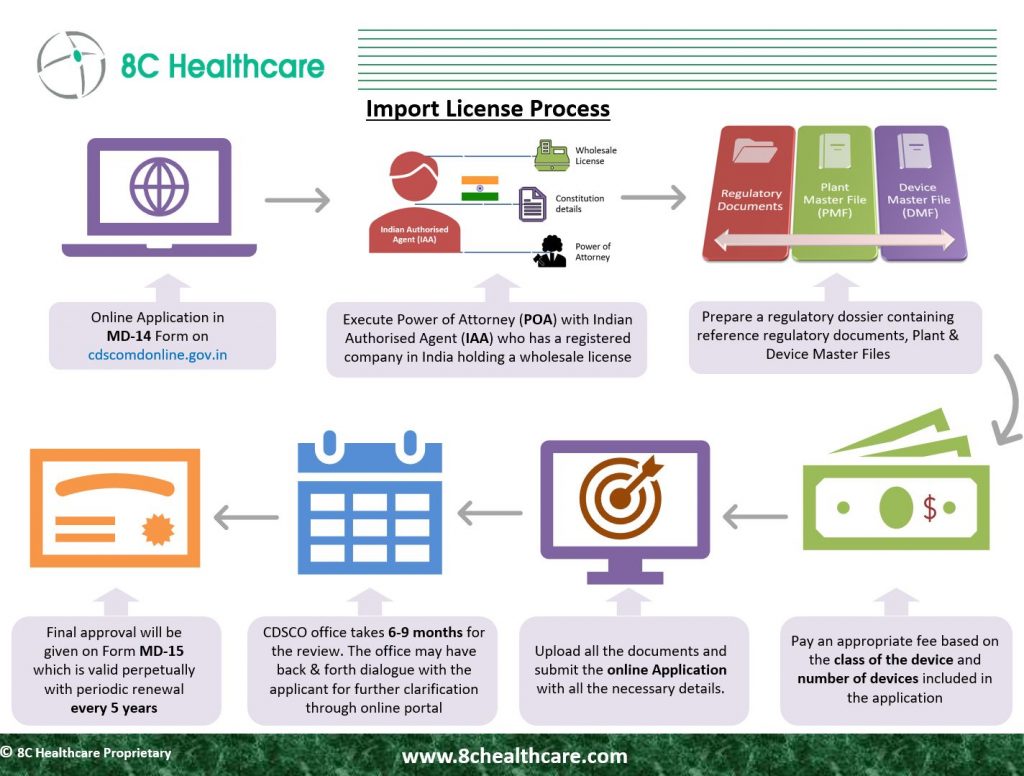
Process for obtaining import license from CDSCO, India
According to the Medical Devices Rule of 2017, the import of notified medical devices necessitates the acquisition of an import license, denoted as Form MD-15, from the CDSCO. Prior to importing these devices, both the manufacturing site and the medical devices themselves must be registered with the CDSCO. The import license for medical devices can be acquired by completing Form MD-14 and submitting it to the CDSCO.
Determine the risk class of medical device or Invitro Diagnostic Device
Categorize medical devices and IVDs according to their intended purpose, potential risks, and the extent of contact with the human body. Determine whether the device falls into Class A (low risk), Class B (low to moderate risk), Class C (moderate to high risk), or Class D (high risk) based on its characteristics.
Grouping of medical devices and IVDs
You can streamline the application process for medical devices that have the same or similar intended purposes or share technological features by combining them into a single application. Assess whether components and variations can be organized into a Family, Group, or System for a more efficient evaluation.
Explore the comprehensive article about the classification and consolidation of medical devices and IVDs in India by clicking this link
Appoint an Indian Authorized Representative
For this purpose, the appointment of an accredited Indian agent is mandatory. This appointed agent should hold a valid license for either manufacturing (for sale or distribution) or wholesale distribution, in accordance with CDSCO guidelines as specified in FORM 20B and FORM 21B. The agent is responsible for initiating the application process to secure an import license for medical devices through the Sugam online portal
For in-depth information about the Indian Authorized Agent or Representative, please refer to this resource.
Prepare Submission Documentation
Assemble the submission package, which includes regulatory and administrative documents, as well as the Plant Master File and Device Master File. Some documents may necessitate both Apostillation and notarization.
In addition to the application for a medical device import license, foreign manufacturers are required to furnish a range of documents that serve as evidence of their compliance with regulatory standards in their country of origin, as well as in GHTF countries, which encompass the United States, Europe, Canada, Australia, and Japan. These countries have well-established medical device regulations, facilitating the CDSCO’s efforts to streamline the regulatory landscape for medical devices in India.
These documents must be currently valid (at least within the last 6 months) and should be legally authenticated, either through notarization, Apostille, or both, before submission. Being aware of these requirements early in the submission process is crucial for effective planning and adhering to submission deadlines.
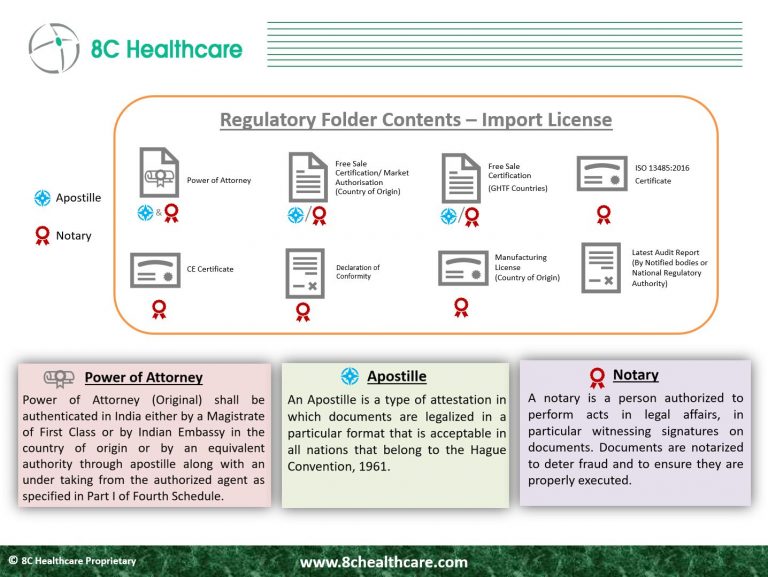
Read more about Plant Master File and Device Master File.
CDSCO review of the import license application
Review of the import license application by CDSCO Upon receipt, the CDSCO will assess the application and, if necessary, request additional information or pose queries. The standard processing timeline for import applications at CDSCO typically ranges from 6 to 9 months.
CDSCO approval
After confirming the accuracy and completeness of the device information, CDSCO grants approval for the import of medical devices in the form of Form MD 15. This import license has a continuous validity, meaning it remains in effect indefinitely as long as the prescribed license retention fee is paid at scheduled intervals, as outlined in the Second Schedule. The license retention fee should be submitted before the completion of each 5-year period from the date of license issuance, unless the Central Licensing Authority suspends or cancels it.
CDSCO Charges for Import License Application Review
The applicant is required to submit distinct fees for both the manufacturing site and the medical devices. The fees differ based on the risk class under which these devices are categorized.
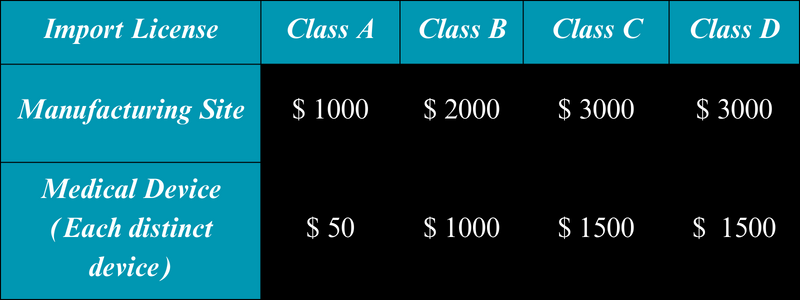
Moreover, the fees for In Vitro Diagnostics (IVDs) and medical devices vary.
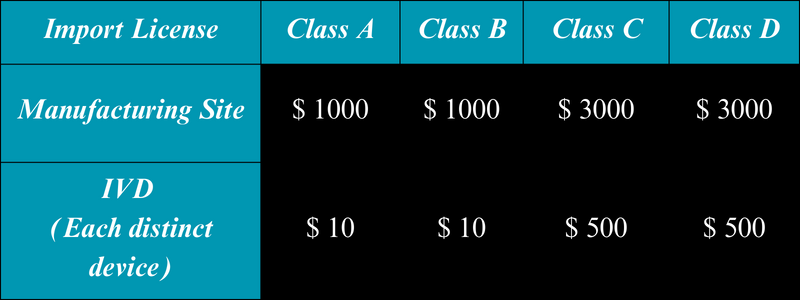
The retention fees for the import license, which are due every 5 years, remain consistent with the initial fees.
Navigating the regulatory landscape for importing medical devices and In Vitro Diagnostics in India is a multifaceted process, with considerations ranging from classification and grouping to fees and retention. Understanding the requirements and adhering to the guidelines set by the CDSCO is crucial for manufacturers and importers to ensure a smooth entry into the Indian market.
If you have any questions or require assistance with the import of medical devices and IVDs in India, feel free to reach out to us at 8Chealthcare. Our experts are here to provide guidance, answer your queries, and help you with the intricacies of regulatory compliance. Contact us at contact@8chealthcare.com to get started on your journey to successfully bring your medical devices and IVDs to the Indian market. “Your success is our Future”.